
What is IQVIA patient portal?
The IQVIA Patient Portal is a global web-based solution built on a Salesforce platform to increase clinical trial patient engagement and retention by enabling transparency and collaboration before, during and after a clinical trial.
Where can I download the IQVIA study Hub app?
This app is available only on the App Store for iPhone and iPad. The IQVIA Study Hub app supports your clinical trial journey by providing a platform to interact with study team members, view upcoming visits, complete eDiaries, track study progress, access study related documents and tap into 24/7 support.
What data does study hub collect for my clinical trial?
Study Hub may also securely collect data from certain approved connected devices used in your clinical trial. If the trial requires and is configured to collect data from Apple Watch, Study Hub uses Apple HealthKit to collect heart rate data so that the primary investigator or study personnel can review and monitor it.
How do I contact IQVIA customer service?
Please contact your IQVIA Account Manager if you have any questions. Good to go!! For technical assistance or inquiries about accessing services on the IQVIA Customer Portal, please email us at [email protected] or submit it online, via Ask IQVIA.
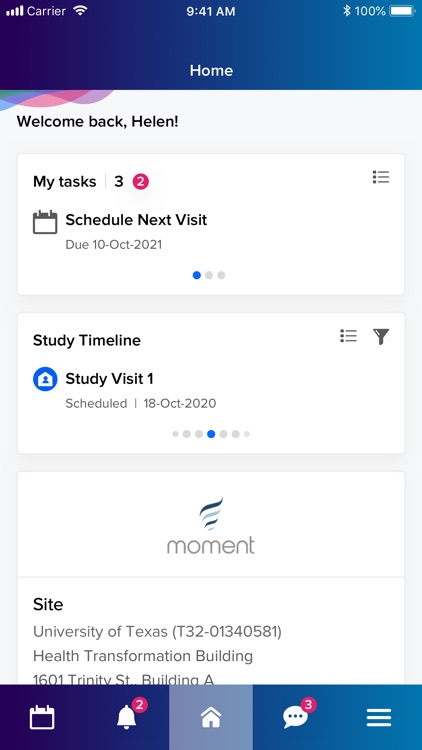
What is IQVIA study hub?
When approved for use in a clinical study, IQVIA Study Hub supports a patient’s clinical trial experience from start to finish by organizing trial activities, sending important reminders, and offering access to support staff – ultimately focused on making clinical trial participation easier and less time-consuming in one platform.
Does Study Hub collect data from Apple Watch?
Study Hub may also securely collect data from certain approved connected devices used in your clinical trial. If the trial collects data from Apple Watch, Study Hub uses the Apple HealthKit to collect the necessary data.
Smarter ways to generate evidence
The increasing costs of developing treatments requires smarter approaches. Access a wide spectrum of data and sourcing approaches from existing secondary data, primary data, registry collaborations, and patient reported outcomes.
Smarter study approaches
We are reimagining evidence generation with innovative approaches you can leverage right now, today.
Accelerated recruitment
By connecting with existing real world data sources, we can more easily find the right patients and quickly move to recruitment.
Regulatory knowledge
One potential challenge of new study designs is regulatory acceptance. IQVIA brings a history of EMA-accepted Drug Utilization Studies (DUS) in multiple therapy areas and geographies and extensive experience working with the FDA, ENCePP, EUnetHTA , MHRA.
Put sites first and be a sponsor of choice
Communication, collaboration and transparency between sites and sponsors mean trials start up and close out faster.
Meet Clinical Trial Challenges Head On
Principal investigators (PIs) engage in clinical research to help their patients survive and thrive with the most advanced science and treatments. But PIs and their staff can quickly become overburdened with paperwork.
Investigator Site Portal
Start, conduct, and complete all your trials more effectively with IQVIA Technologies’ Investigator Site Portal, a collaborative platform designed to make life easier for sites and improve data quality.
